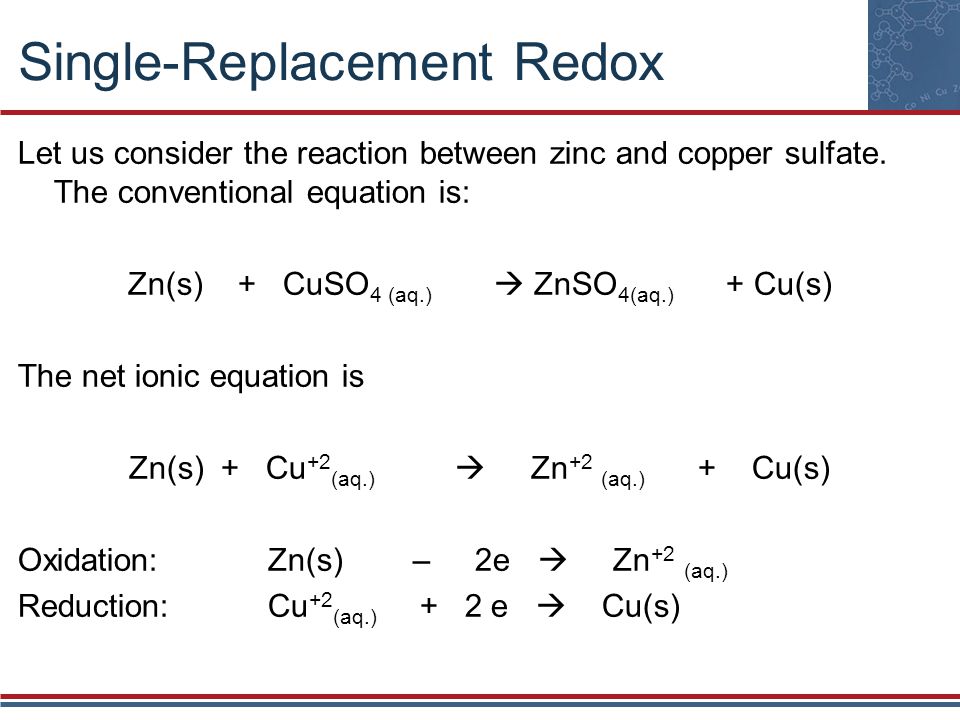
Copper Sulfate + Zinc Metal . what happens when zinc reacts with copper sulphate solution? However, in the second reaction, the zinc ion is not. in the first reaction, the copper ion is able to oxidize the zinc metal. Zinc is a more reactive. zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of. it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc (zn) is more reactive than copper (cu), therefore it can. important tips for balancing chemical equations like zinc plus copper (ii). when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution.
from fity.club
in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. Zinc (zn) is more reactive than copper (cu), therefore it can. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of. important tips for balancing chemical equations like zinc plus copper (ii). it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. in the first reaction, the copper ion is able to oxidize the zinc metal. what happens when zinc reacts with copper sulphate solution? Zinc is a more reactive. However, in the second reaction, the zinc ion is not.
Reaction Of Zinc And Copperii Ion Chemdemos
Copper Sulfate + Zinc Metal Zinc is a more reactive. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. important tips for balancing chemical equations like zinc plus copper (ii). Zinc is a more reactive. it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. in the first reaction, the copper ion is able to oxidize the zinc metal. Zinc (zn) is more reactive than copper (cu), therefore it can. what happens when zinc reacts with copper sulphate solution? in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of. However, in the second reaction, the zinc ion is not.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Copper Sulfate + Zinc Metal it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. important tips for balancing. Copper Sulfate + Zinc Metal.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Copper Sulfate + Zinc Metal it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc (zn) is more reactive than copper (cu), therefore it can. what happens when zinc reacts with copper sulphate solution? in this video, you will observe the displacement of. Copper Sulfate + Zinc Metal.
From fity.club
Reaction Of Zinc And Copperii Ion Chemdemos Copper Sulfate + Zinc Metal Zinc (zn) is more reactive than copper (cu), therefore it can. in the first reaction, the copper ion is able to oxidize the zinc metal. what happens when zinc reacts with copper sulphate solution? it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental. Copper Sulfate + Zinc Metal.
From www.youtube.com
Zinc Plating on Copper Metal YouTube Copper Sulfate + Zinc Metal in the first reaction, the copper ion is able to oxidize the zinc metal. it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one. Copper Sulfate + Zinc Metal.
From uwaterloo.ca
Zinc metal in a solution of copper(II) sulfate and sulfuric acid from Copper Sulfate + Zinc Metal Zinc (zn) is more reactive than copper (cu), therefore it can. in the first reaction, the copper ion is able to oxidize the zinc metal. in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. what happens when zinc reacts with copper sulphate solution? However, in the second reaction, the zinc. Copper Sulfate + Zinc Metal.
From www.dreamstime.com
CopperII Sulfate and Zinc Powder in Test Tube with Plug Cap. Cosmetic Copper Sulfate + Zinc Metal zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of. in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. what happens when zinc reacts with copper sulphate solution? it is commonly known that when zinc. Copper Sulfate + Zinc Metal.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0148 Science Copper Sulfate + Zinc Metal important tips for balancing chemical equations like zinc plus copper (ii). it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc is a more reactive. Zinc (zn) is more reactive than copper (cu), therefore it can. zn +. Copper Sulfate + Zinc Metal.
From www.sciencephoto.com
Nickel Reacting With Copper Sulfate Stock Image C028/0162 Science Copper Sulfate + Zinc Metal when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. important. Copper Sulfate + Zinc Metal.
From www.alamy.com
Solid metallic copper at bottom of glass beaker containing Zinc Copper Sulfate + Zinc Metal in the first reaction, the copper ion is able to oxidize the zinc metal. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. important tips for balancing chemical equations like zinc plus copper (ii). Zinc (zn) is more reactive than copper. Copper Sulfate + Zinc Metal.
From brainly.com
Mindy places a strip of zinc in a copper sulfate solution, as shown in Copper Sulfate + Zinc Metal zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of. Zinc (zn) is more reactive than copper (cu), therefore it can. Zinc is a more reactive. However, in the second reaction, the zinc ion is not. important tips for balancing chemical equations like zinc. Copper Sulfate + Zinc Metal.
From www.youtube.com
Copper Sulfate on all metals YouTube Copper Sulfate + Zinc Metal Zinc is a more reactive. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. important tips for balancing chemical equations like zinc plus copper (ii). Zinc (zn) is more reactive than copper (cu), therefore it can. zn + cuso4 = cu. Copper Sulfate + Zinc Metal.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Copper Sulfate + Zinc Metal in the first reaction, the copper ion is able to oxidize the zinc metal. what happens when zinc reacts with copper sulphate solution? However, in the second reaction, the zinc ion is not. in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. zn + cuso4 = cu + znso4. Copper Sulfate + Zinc Metal.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper Sulfate + Zinc Metal when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a. Copper Sulfate + Zinc Metal.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate, 2 of 7 Stock Image C028/0140 Copper Sulfate + Zinc Metal in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole. Copper Sulfate + Zinc Metal.
From www.toppr.com
What happens when a piece of silver metal is added to the copper Copper Sulfate + Zinc Metal However, in the second reaction, the zinc ion is not. what happens when zinc reacts with copper sulphate solution? in the first reaction, the copper ion is able to oxidize the zinc metal. in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. zn + cuso4 = cu + znso4. Copper Sulfate + Zinc Metal.
From melscience.com
Reaction between copper sulfate and iron MEL Chemistry Copper Sulfate + Zinc Metal However, in the second reaction, the zinc ion is not. important tips for balancing chemical equations like zinc plus copper (ii). it is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. in this video, you will observe the displacement. Copper Sulfate + Zinc Metal.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Copper Sulfate + Zinc Metal in this video, you will observe the displacement of zinc metal from copper (ii) sulfate solution. what happens when zinc reacts with copper sulphate solution? when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. it is commonly known that when. Copper Sulfate + Zinc Metal.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Copper Sulfate + Zinc Metal zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of. when a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc. Zinc (zn) is more reactive than copper (cu), therefore. Copper Sulfate + Zinc Metal.